Research profile
The main research objective of the Department is to increase knowledge and understanding of the mechanisms of biological (antitumor and antibiotic) action of metallodrugs and to use this enhanced knowledge to develop new classes of metallodrugs with truly novel mechanisms of action and novel spectra of biomedical activity.
The methods, which we had available in the last period, enabled us to obtain a more complex view on the mechanism of action of new metallodrugs based on a combination of information obtained on molecular and cellular levels.
Major methods
Molecular biophysics, biochemistry and molecular pharmacology:
- DNA binding studies, polarography, FAAS, absorption spectrophotometry.
- Sequence specificity of DNA binding.
- Binding to DNA with different G+C content and synthetic DNAs.
- HPLC analysis of DNA enzymatically hydrolyzed to nucleosides.
- Replication and transcription mapping.
- EtBr, fluorescent probe for crosslinking, interstrand crosslinking assays.
- Conformational analysis of DNA modified by metal complexes.
- DNA melting, microcalorimetry (DSC, ITC), thermophoresis.
- CD, LD spectroscopy, Raman spectrophotometry, spectrofluorimetry, AFM.
- Supercoiled plasmid DNA unwinding by gel electrophoresis.
- Chemical probes of DNA conformation.
- DNA bending and unwinding by single, site-specific metal adducts.
- Translesion DNA and RNA synthesis and replication bypass.
- Recognition of DNA modified by metal complexes by specific proteins.
- Hydroxyl radical, DNase I footprinting.
- Nucleotide excision repair of DNA, DNA repair synthesis.
Cellular pharmacology assays:
- Cytotoxicity in cell cultures (including cancer stem cells and 3D spheroids) and single cell gel electrophoresis (comet) assay.
- Flow cytometric analysis of the cell cycle.
- Synthesis of DNA, RNA and proteins in cells.
- Apoptosis, necrosis and autophagy induction.
- Peptide mass fingerprinting, Western blotting.
- Mutation-assays.
- Real-time quantitative PCR.
- Cellular drug uptake by FAAS and ICP-MS.
- Localization studies by confocal laser microscopy.
- Real-time cell electronic sensing.
- Photoxicity testing.
- Determination of reactive oxygen species.
- Measurements of mitochondrial transmembrane potential, invasion, migration, and readhesion in tumor cells.
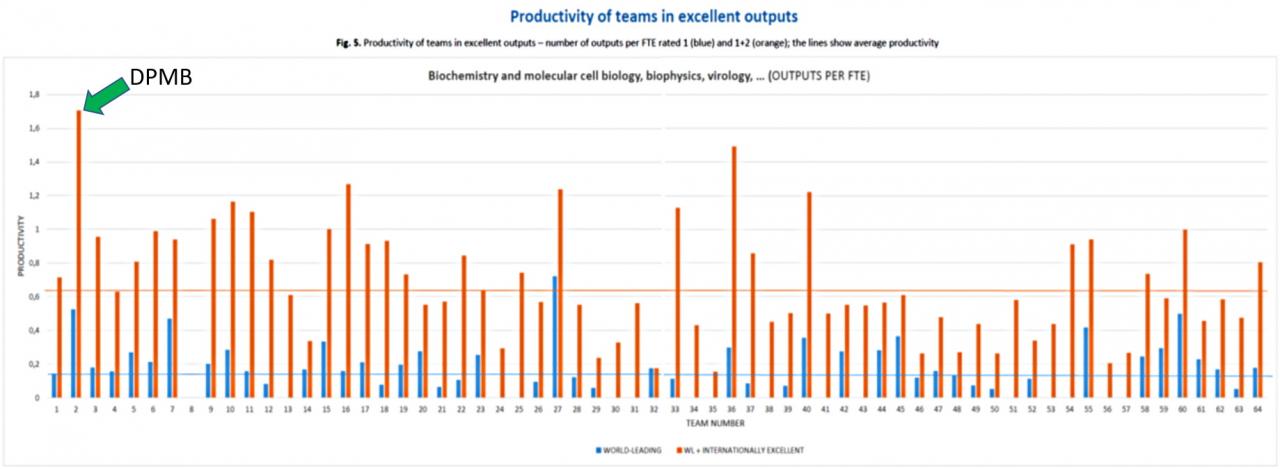
The department (DPMB) was evaluated in the I. phase of this evaluation in 2021 as the most productive team in excellent outputs among 64 teams of the Academy of Sciences in the field of Biochemistry, Molecular and cell Biology, Biophysics, Virology …