Výzkumné zaměření
Hlavním cílem výzkumu na oddělení je rozšířit znalosti a porozumění mechanismu biologického (protinádorového a antibiotického) působení léčiv na bázi kovů a využít těchto znalostí k vývoji nových tříd metalofarmak s inovativním mechanismem působení a novým spektrem biomedicinální aktivity.
Metody, které byly v poslední době na oddělení zavedeny, umožňují získat komplexnější pohled na mechanismus působení nových metalofarmak, založený na kombinaci informací ze studií na molekulární i buněčné úrovni.
Hlavní metody
Molekulární biofyzika, biochemie and molekulární farmakologie:
- Studie vazby na DNA: polarografie, FAAS, absorpční spektrofotometrie.
- Sekvenční specificita vazby na DNA.
- Vazba na DNA s rozdílným obsahem G+C and syntetické DNA.
- HPLC analýza DNA enzymaticky hydrolyzované na nukleosidy.
- Replikační and transkripční mapování.
- EtBr, fluorescenční sonda pro tvorbu můstků v DNA, kvantitativní stanovení meziřetězcových můstků.
- Konformační analýza DNA modifikované komplexními sloučeninami kovů.
- Tání DNA, mikrokalorimetrie (DSC, ITC), termoforéza.
- CD, LD spektroskopie, Ramanova spektrofotometrie, spektrofluorimetrie, AFM.
- Rozvíjení superhelikální plasmidové DNA sledované gelovou elektoforézou.
- Chemické sondy konformace DNA.
- Ohyby a rozvíjení DNA šroubovice vyvolané jedním místně-specifickým aduktem daného metalofarmaka.
- Translézová syntéza DNA a RNA a replikační překlenutí lézí.
- Rozlišení DNA modifikované komplexy kovů specifickými proteiny.
- „Footprinting“ pomocí hydroxylových radikálů nebo DNázy I.
- Nukleotidová excizní oprava DNA, reparační syntéza DNA repair.
Metody buněčné farmakologie:
- Cytotoxicita (antiproliferativní aktivita) v buněčných kulturách (včetně nádorových kmenových buněk a 3D sferoidů).
- Elektroforéza jedné buňky („kometový test“).
- Průtoková cytometrie – analýza buněčného cyklu.
- Syntéza DNA, RNA and proteinů v buňkách.
- Indukce apoptózy, nekrózy, autofagie a dalších typů buněčné smrti.
- Western blotting.
- Mutační analýza.
- Kvantitativní PCR v reálném čase.
- Průnik látek do buněk a akumulace v buňkách pomocí FAAS a ICP-MS.
- Lokalizační studie konfokální laserovou mikroskopií.
- Real-time cell electronic sensing.
- Testy fototoxicity.
- Stanovení vzniku reaktivních sloučenin kyslíku.
- Měření mitochondriálního membránového potenciálu, invazivity, migrace a readheze nádorových buněk.
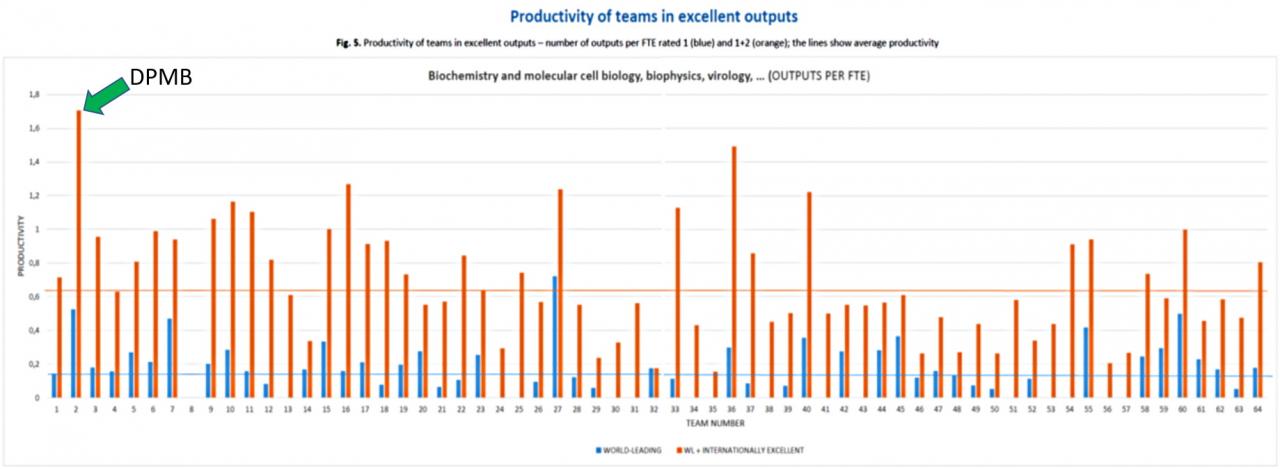
Oddělení Molekulární biofyziky a farmakologie (DPMB) bylo v první fázi hodnocení pracovišť AV v roce 2021 vyhodnoceno jako nejproduktivnější tým v excelentních výstupech z celkem 64 hodnocených týmů Akademie věd v oboru Biochemie, molekulární a buněčná biologie, biofyzika, virologie…