Gabriela Ambrožová, Mgr., Ph.D.
Kristýna Turková, Mgr., PhD.
Anna Kocurková, Mgr.
Miriam Sandanusová, Mgr.
Martin Šindelář, Mgr.
Eva Pecháčková,Bc.
Myeloid cells and regulation of their functions
Myeloid cells represent a heterogeneous group of blood cells, responsible for, besides other functions, for triggering and regulating immune response and the subsequent inflammatory process. While acute inflammation is directed toward invading pathogens and injured cells, thus enabling tissue regeneration and wound healing; chronic inflammation can lead to severe tissue dysfunction and pathologies including cardiovascular, autoimmune, and neurodegenerative diseases. The aim of the project is to clarify effects of myeloid cells in the regulation of inflammation, its initiation and progression, focusing especially on different functional phenotypes of neutrophils, the most abundant circulating leukocytes, forming the first line of defense but also contribute to concomitant host tissue damage. Traditionally viewed as a homogenous population, a growing body of evidence challenges this paradigm; suggesting the existence of distinct neutrophil subpopulations with different regulatory roles. Dynamic changes of neutrophil subpopulations and their phenotype in relation to their immuno-regulatory properties are analyzed within the project, while advanced molecular methods (e.g. spectral flow cytometry) and mathematical approaches (e.g. computational biochemical models) are applied.
Obtained data are of crucial importance in the better understanding of neutrophil immuno-regulatory roles, particularly in chronic inflammatory diseases and in development of new therapies that could target pathogenic neutrophil subpopulations without compromising the beneficial ones.
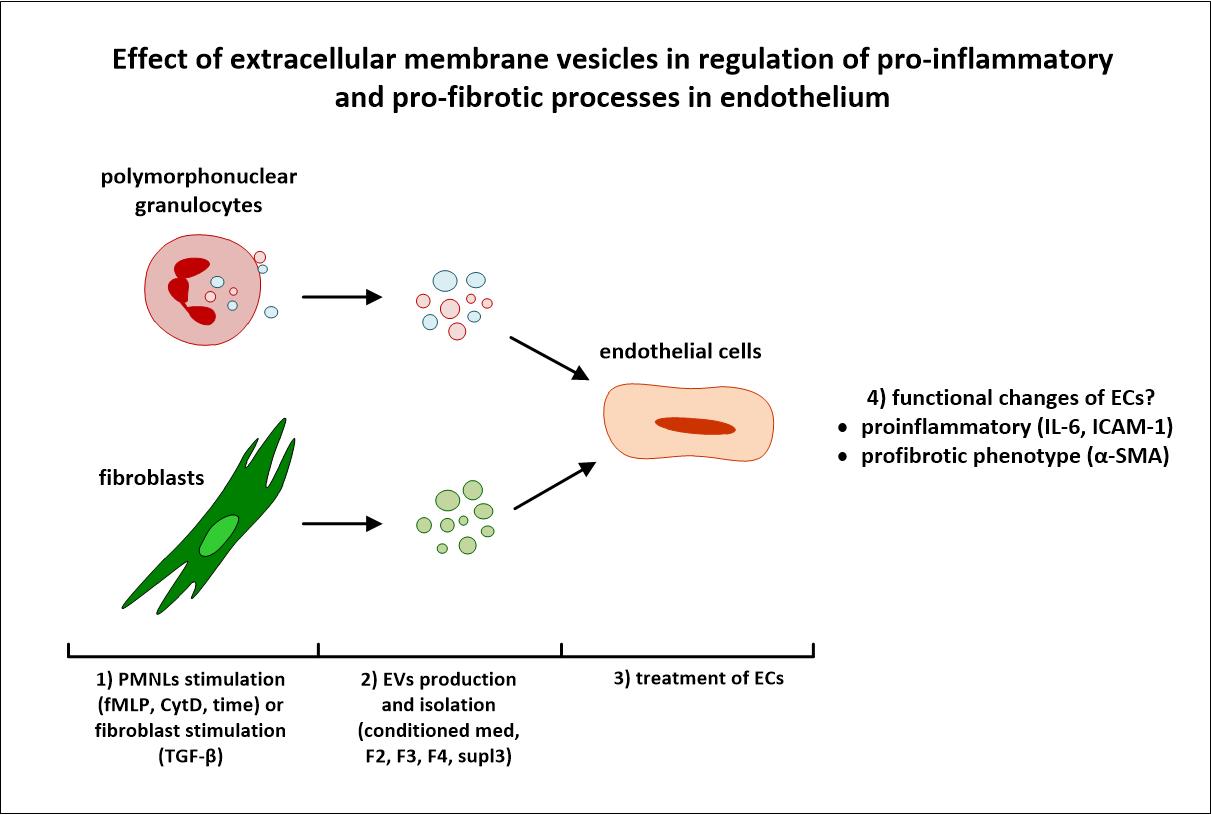
Immuno-regulatory role of neutrophil-released extracellular membrane vesicles in vascular inflammation
Chronic inflammation initiates and promotes a wide variety of pathological processes, including cardiovascular diseases such as atherosclerosis. Prominent among pro-inflammatory stimuli in myeloid cells are extracellular membrane vesicles (EMVs), that arise from cells and circulate systemically, modulating the behaviour or functional capacity of the target cells with which they associate.
Our project focuses its attention on the characterization of the role of EMVs derived from neutrophils; such leukocytes involved in the first phase of inflammatory response in the pathogenesis of cardiovascular diseases. Different types of EMVs are isolated using ultracentrifugation and their composition, phenotype, and functional capacity are characterized. Furthermore, we assess how neutrophil-derived EMVs modulate the behaviour of endothelial cells, fibroblasts, monocytes and macrophages, the cell types that conspicuously participate in cardiovascular disease development.
Development of novel therapeutics based on hyaluronan for treatment of inflammation-related disorders
Hyaluronan (HA), one of body’s own biopolymers, is often used as a basis for many bioactive materials and pharmaceuticals. Itself, it is able to promote healing and modulate inflammation. However, to enhance its efficacy, this highly soluble and rapidly degradable polymer can be stabilized. Newly synthetized derivatives have acquired not only a range of physicochemical properties but also novel specific biological properties. These biopolymers are able to induce, reduce or otherwise modulate inflammation; acute in the case of acute wounds or surgical interventions; as well as chronic inflammation that often accompanies a variety of pathologies including non-healing wounds and intestinal inflammatory diseases.
The main goal of the project is to identify new mechanisms and principles of HA actions in the course of healing and inflammatory processes that will be used for development of HA based pharmaceuticals and biomaterials. Biological properties of HA derivatives are characterized within the context of inflammatory processes, which allows further optimization of these derivatives in terms of their molecular weight, the degree of substitution or contents of specific substituents and of course, stability and biodegradability. For the purpose of screening the capacity of HA derivatives to modulate innate and specific immune responses, selected in vitro assays using human leukocytes or mouse leukocytes and lymphatic tissues are used. Furthermore, the efficacy and mechanism of action of the most promising candidates are studied in vivo.
Our project should provide detailed information about effects of newly synthetized HA derivatives on immune cells, revealing their potential to affect inflammatory response and the pathological consequences, thereby helping to develop efficient state-of-the-art drugs and biomaterials in the treatment of inflammatory diseases.

